to view content for your location.


Quality and durability of our machines is always the highest priority at Kuhner. Kuhner products have proven their value worldwide under GMP requirements. Documentation, development and production along with the full life cycle of our products are continuously enhanced to match the requirements of 21 CFR and GAMP 5.
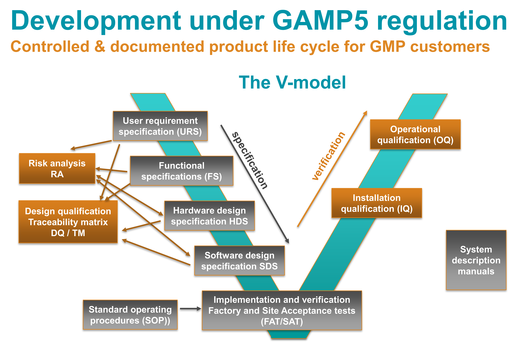

By implementing the GAMP 5 V model, Kuhner was able to completely fulfill the increasing requirements of the pharmaceutical industry concerning GMP related qualification of our lab equipment. Our GMP customers worldwide recognizes Kuhner as a reliable business partner who can also accommodate custom projects.
Kuhner offers the entire set of qualification documents (IQ/OQ) for their shakers, bioreactor systems, software and communication interfaces. All documents are available as Kuhner standard documents or can be adapted to customer’s requirements in format, layout and scope. Based on customer feedback, we continuously enhance the documents to respond to future requirements and changing regulations.
We also extend the service of executing the IQ/OQ equipment qualification at the customer’s site.
